The FAIR project aims at evaluating the activation of the innate immune system in the airways as an alternative adjunct strategy to standard of care antibiotics for treating pneumonia caused by antibiotic-resistant bacteria
Recent progress in understanding host-pathogen interactions indicates that innate immunity can be induced to produce an array of antimicrobial effectors for rapid defence against a broad spectrum of pathogenic microorganisms - including AMR bacteria. Possible new treatments would include the combination of an agonist of innate immunity (i.e. an immunomodulatory agent) with antibiotics, as a promising way of boosting effectiveness and overcoming AMR. Our consortium has proved the concept whereby the respiratory tract administration of a Toll-like receptor 5 (TLR5) agonist, the immunomodulatory flagellin FliC∆174-400, enhances the therapeutic outcome of antibiotic in the context of pneumonia caused by S. pneumoniae.
The FAIR proposal’s main objectives are to:
- develop a unique approach: aerosol delivery of the immunomodulatory flagellin, achieving direct release into the airways, prompting innate immunity activation in the lungs, and preventing unwanted systemic immune activation.
- prove the synergy between nebulized flagellin and antibiotics in the treatment of antibiotic-resistant pneumonia in physiologically relevant animal models, and human-cell-based models of the lung airway epithelium.
- identify specific biomarkers of TLR5 signalling and augmented protection in inflamed airways and the blood of preclinical models.
- build a translational modelling and simulation platform to optimize experimental design and characterize the pharmacokinetics/pharmacodynamics and safety margins of nebulized flagellin
- evaluate the safety of a single nebulized dose of flagellin in a Phase I clinical trial in healthy participants.
- discover stratification markers in pneumonia patients and thus define the target population for flagellin-based adjunct therapy.
- examine the adjunct therapy’s acceptability for patients and health care professionnals.
- use health economic modelling to assess the potential for nebulized flagellin as a cost-effective, affordable adjunct to conventional treatment of AMR pneumonia in European healthcare systems.
As a measurable, realistic and achievable project, FAIR’s strengths are as follows:
- The immediate availability of a toxicology grade batch of flagellin, which is produced through a EU TRANSVAC2 grant.
- The immediate availability of the Aerogen® Solo nebulizer.
- The existing data and blood samples from patients with pneumonia in the MARS cohort.
- The construction of communication/dissemination partnerships with patient associations, and EU initiatives on AMR
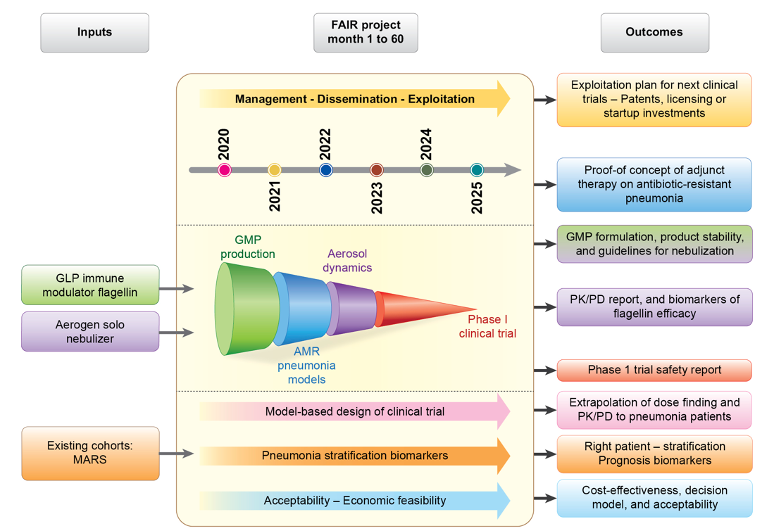
The project will last for 60 months involving 11 partners, including 6 research centres, 2 SMEs, 3 hospitals, 1 patient and healthcare professional organisation, and 1 technology transfer company in 7 countries. An overview of the timeline, major tasks and the flow to bring innovation to the clinic is given here.
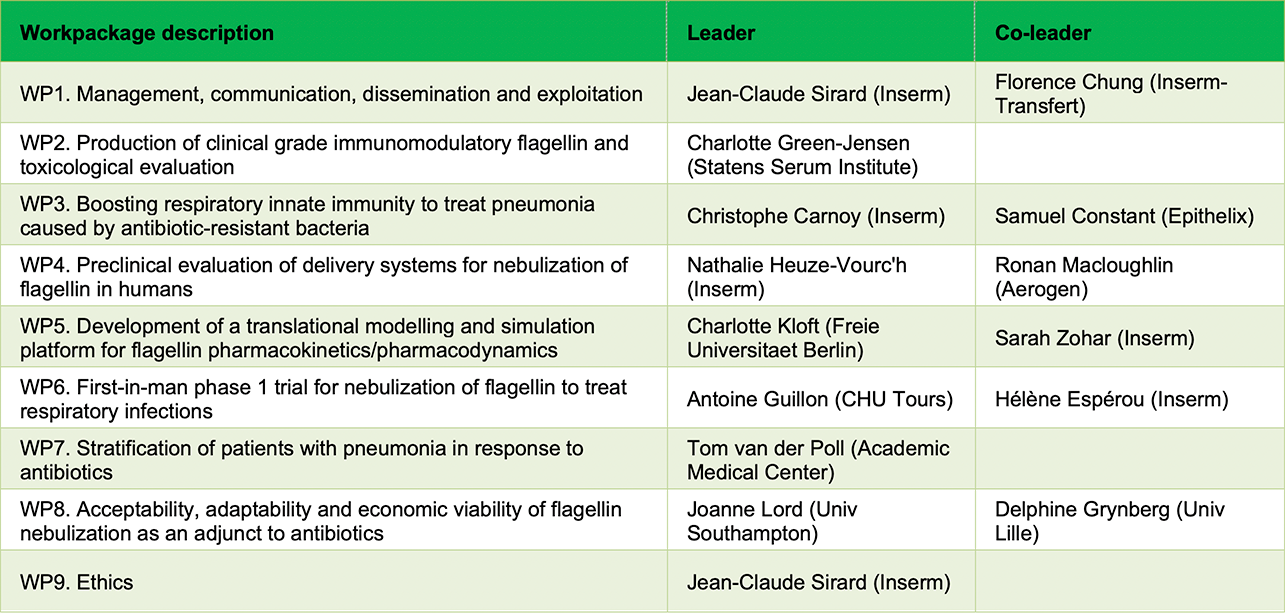
The FAIR consortium is organized as follows:
The FAIR project expects to demonstrate the relevance and safety of the nebulized flagellin approach in humans in a phase I trial. From then on, the intention is to rapidly progress flagellin therapy via further clinical development in pneumonia patients. This approach will increase robustness of data, bring innovation to patients and provide evidence to impact clinical practice and improve patient care. The FAIR exploitation plan will enable (i) the straightforward development of a drug-device combination, and (ii) the identification of biomarkers of value for patient stratification based on the clinical outcome, and that can be used to optimize the design of future clinical trials of flagellin. FAIR will develop new avenues of research on mechanisms of action of the adjunct flagellin, in order to better monitor the drug’s biological effects and effectiveness. Lastly, FAIR will determine whether the treatment is acceptable for pneumonia patients and is cost-effective for the health economy.
The FAIR project expects to see direct impacts on:
- patients and clinicians: meeting a medical need by improving clinical care, social functioning, and better quality of life
- society in general: longer-term reductions in the cost and global burden of pneumonia
- research: new avenues for antibiotics in the context of immunity, pharmacokinetics/pharmacodynamics modelling of nebulized biologics and its extrapolation to patients, and socio-economic assessments of alternative treatment approaches.
- industry: the exploitation of nebulizers, drug formulations, cell-based assays, and prognostic tools for identifying poor responders to standard treatments.

